JEE 2006 Sample Question Paper : Page 1�|�Page 2�|�Page 3�|�Page 4�|�Page 5�|�Page 6�|�Page 7
IIT JEE 2006 Sample Question Paper
Passage 4
The following table represents the concentration of ions and dissolved gases in the sediment at the bottom of an ocean. A depth of 0 centimeters (cm) represents the top of the sediment. The concentrations are expressed in parts per million (ppm). The acidity of a solution is represented on a scale known as pH. A pH of 1 is very acidic, a pH of 7 is neutral, and a pH of 14 is very basic.
| Depth (cm) |
Temperature (oC) |
pH | Concentration in sediment (ppm) | |||||
|---|---|---|---|---|---|---|---|---|
| SO42- | S2- | CO2 | Fe3+ | Fe2+ | O2 | |||
| 0 | 4 | 7.0 | 7.0 | 0.0 | 1.0 | 4.0 | 0.5 | 2.0 |
| 5 | 5 | 6.5 | 5.0 | 2.0 | 1.5 | 3.0 | 1.5 | 1.0 |
| 10 | 7 | 6.0 | 3.5 | 3.5 | 2.0 | 2.0 | 2.0 | 0.0 |
| 15 | 9 | 5.5 | 3.3 | 3.8 | 3.0 | 0.8 | 3.8 | 0.0 |
| 20 | 10 | 5.0 | 3.0 | 4.0 | 1.0 | 0.5 | 4.0 | 0.0 |
Q. 16 According to the information provided in the table, the concentration of which of the following ions and dissolved gases is constant for sediment depths of 10 cm or more?
- Sulfide (S2-)
- Carbon dioxide (CO2)
- Ferric iron (Fe3+)
- Oxygen (O2)
Q. 17 The graph below best represents the relationship between concentration and sediment depth for which of the following ions and dissolved gases?
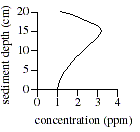
- Ferrous iron (Fe2+)
- Oxygen (O2)
- Carbon dioxide (CO2)
- Sulfate (SO42-)
Q. 18 If the trends indicated in the table were to continue, one would predict the pH of the sediments at a depth of 35 cm to be:
- 1.5.
- 3.5.
- 4.5.
- 6.0.
Q. 19 A certain type of bottom-dwelling microorganism thrives under the following environmental conditions: low concentrations of Fe2+, high concentrations of O2, and a neutral pH. Based on the table, at which of the following sediment depths would one most likely find this microorganism?
- 0 cm
- 5 cm
- 10 cm
- 15 cm
Q. 20 A researcher wants to determine whether an unidentified sediment sample was drawn from a depth of 15 cm or 20 cm. Based on the information in the table, which of the following would NOT confirm the depth of the sample?
- O2 concentration
- Fe3+ concentration
- S2- concentration
- pH

